Abstract
Introduction
Following treatment with a bypassing agent, parents or caregivers often face difficulties in determining bleeding episode (BE) resolution in children with hemophilia A or B and inhibitors (CwHABI), potentially contributing to a longer treatment duration in children as compared to adults (Valentino et al, Haemophilia 2012; 18:554-60. Gruppo et al, Haemophilia 2013; 19:524-32). Eptacog beta is a new recombinant activated human factor VII proven to be safe and effective for the treatment and control of BEs in patients with hemophilia A or B with inhibitors (≥12 years of age). The pivotal phase 3 trial (PERSEPT 1; NCT02020369) included subjects from ages 12 to <18 years, in addition to adult subjects. A subsequent phase 3 trial (PERSEPT 2; NCT02448680) further examined the safety and efficacy of eptacog beta for bleed treatment in CwHABI <12 years of age. Within this population, we hypothesized that caregivers could better ascertain treatment success in older children, which would manifest as increasing eptacog beta efficacy measurements and tighter 95% confidence intervals (CIs) with increasing subject age. We explored this question by analyzing BE treatment success in three age subgroups within PERSEPT 1 and PERSEPT 2.
Aims
The study objective is to evaluate and compare the clinical response to eptacog beta for BEs in CwHABI at 12 and 24 hours after initial dose of eptacog beta in 3 pediatric age subgroups (<6 years, 6 to <12 years, and 12 to <18 years).
Methods
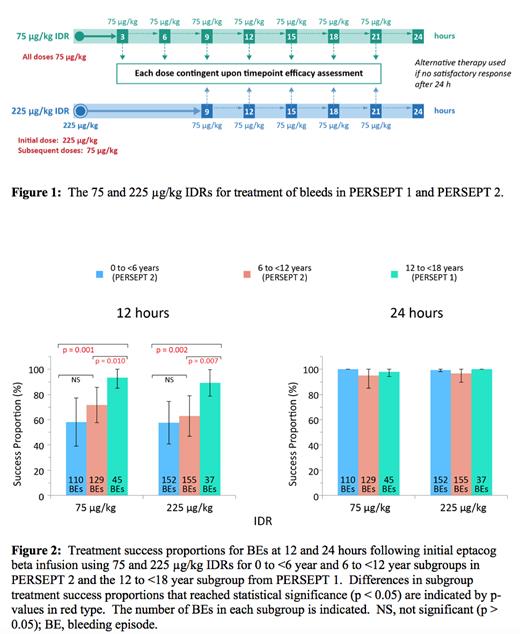
PERSEPT 1 and PERSEPT 2 were prospective, global, open-label trials of eptacog beta using two initial dose regimens (IDRs) of 75 and 225 µg/kg in a randomized, non-blinded, crossover design. Subjects received initial doses of 75 or 225 µg/kg eptacog beta followed by 75 µg/kg dosing at predefined intervals (determined by clinical response) to treat BEs (Figure 1). Treatment success for mild or moderate BEs was defined as obtaining a hemostasis evaluation of "excellent" or "good" with no use of additional eptacog beta, alternative hemostatic agents or blood products, and no increase in pain following the first "excellent" or "good" assessment. Evaluations were provided by the parent/caregiver in conjunction with the study participant when possible, depending upon subject age and verbal capacity. Written informed consent from the participants' parents/legal guardians were obtained at enrollment.
Results
Thirty subjects were assessed (25 from PERSEPT 2 [13 subjects, 0 to <6 years; 12 subjects, 6 to <12 years] and 5 from PERSEPT 1 [ages 12 to <18 years]). These subjects experienced 628 mild/moderate BEs. No subject was receiving emicizumab prophylaxis. Nearly all BEs in every pediatric age subgroup were successfully treated by 24 hours after initial eptacog beta infusion (Figure 2). At 12 hours, BE treatment success proportions in the 0 to <6 year, 6 to <12 year, and 12 to <18 year subgroups for the 75 µg/kg IDR were 58%, 72%, and 93%, respectively. Corresponding treatment success proportions for the 225 µg/kg IDR in the 0 to <6 year, 6 to <12 year, and 12 to <18 year subgroups were 58%, 63%, and 89%, respectively. The increased treatment success proportions seen for the 12 to <18 year subgroup over those seen for the 0 to <6 year and 6 to <12 year subgroups were statistically significant for both IDRs (p < 0.05; Figure 2). Differences in treatment success between the 0 to <6 year and 6 to <12-year subgroups were not statistically significant for either IDR. Treatment success point estimates at 12 hours in the 0 to <6 years age group showed the widest CIs among the various subgroups (Figure 2).
Conclusions
Eptacog beta treatment of BEs in this pediatric population yielded remarkable (>95%) treatment success proportions in both IDRs by 24 hours after initial eptacog beta infusion of 75 or 225 µg/kg. Differing age group pharmacokinetics could contribute to the observed increase in treatment efficacy at 12 hours with increasing subject age. In addition, when taken together with the wide CIs associated with treatment success point estimates at 12 hours for the 0 to <6 year subgroup, these results are consistent with well-known challenges that drive pediatric dosing of bypassing agents: chiefly, that of caregivers experiencing uncertainty with regard to BE resolution in young children. The high efficacy and narrow 95% CIs seen at 24 hours further indicate that caregivers had achieved clarity regarding BE resolution by the 24-hour timepoint.
Young: Apcintex, BioMarin, Genentech/Roche, Grifols, Novo Nordisk, Pfizer, Rani, Sanofi Genzyme, Spark, Takeda, and UniQure: Consultancy; Genentech/Roche, Grifols, and Takeda: Research Funding. Pipe: Catalyst Biosciences: Consultancy; CSL Behring: Consultancy; HEMA Biologics: Consultancy; Freeline: Consultancy, Other: Clinical trial investigator; Novo Nordisk: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy, Other; Sangamo Therapeutics: Consultancy; Sanofi: Consultancy, Other; Takeda: Consultancy; Spark Therapeutics: Consultancy; uniQure: Consultancy, Other; Regeneron/ Intellia: Consultancy; Genventiv: Consultancy; Grifols: Consultancy; Biomarin: Consultancy, Other: Clinical trial investigator; Bayer: Consultancy; ASC Therapeutics: Consultancy; Apcintex: Consultancy; Octapharma: Consultancy; Shire: Consultancy. Carcao: Bayer, Bioverativ/Sanofi, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, and Shire/Takeda: Research Funding; Bayer, Bioverativ/Sanofi, CSL Behring, Grifols, LFB, Novo Nordisk, Pfizer, Roche, and Shire/Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Castaman: Uniqure: Honoraria; Bayer: Honoraria; Sobi: Honoraria; CSL Behring: Honoraria; Novo Nordisk: Honoraria; Kedrion: Honoraria; LFB: Honoraria; Grifols: Honoraria; Werfen: Honoraria; Biomarin: Honoraria; Sanofi: Honoraria; F Hoffmann-La Roche Ltd: Honoraria. Davis: Genentech, Spark Therapeutics, BioMarin, Bayer: Consultancy; Takeda, Sanofi: Honoraria; Genentech, Sanofi, Novo Nordisk: Membership on an entity's Board of Directors or advisory committees. Ducore: Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; HEMA Biologics: Consultancy, Honoraria; Bayer: Consultancy, Honoraria, Speakers Bureau; Shire: Consultancy, Honoraria. Dunn: Sanofi, Takeda, Freeline, BioMarin, ATHN, Novo Nordisk: Research Funding; Genentech, Kedrion, CSL Behring, BioMarin: Consultancy; UniQure, CSL Behring, World Federation of Hemophilia USA: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Honoraria. Journeycake: HEMA Biologics: Honoraria; LFB: Honoraria. Khan: Genentech, Octapharma, BioMarin, CSL Behring, HEMA Biologics, Kedrion, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Mahlangu: Bayer, Biogen, BioMarin, CSL, Novo Nordisk, Sobi, Roche, and UniQure: Research Funding; Amgen, Bayer, Biotest, Biogen, Baxalta, CSL Behring, Catalyst Biosciences, Novo Nordisk, Roche, and Spark: Membership on an entity's Board of Directors or advisory committees; Alnylam, Bayer, Biotest, Biogen, Novo Nordisk, Pfizer, Sobi, Shire, Roche, ISTH, and WFH: Speakers Bureau. Meeks: Sangamo Therapeutics: Consultancy; Spark Therapeutics: Consultancy; National Hemophilia Foundation: Research Funding; Pfizer: Consultancy; Sanofi: Consultancy; CSL Behring: Consultancy; Genentech: Consultancy; Takeda: Consultancy; Hemophilia of Georgia: Research Funding; National Institutes of Health: Research Funding. Négrier: UniQure: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Biomarin: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche-Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi-Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Spark: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Recht: Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders: Speakers Bureau; uniQure: Consultancy; Takeda: Consultancy; Sanofi: Consultancy; Pfizer: Consultancy; Octapharma: Consultancy; Novo Nordisk: Consultancy; Kedrion: Consultancy; Hema Biologics: Consultancy; Genentech: Consultancy; CSL Behring: Consultancy; Catalyst Biosciences: Consultancy; American Thrombosis and Hemostasis Network: Current Employment; Oregon Health & Science University: Current Employment. Chrisentery-Singleton: Biomarin: Speakers Bureau; Spark: Consultancy, Research Funding; Takeda: Consultancy, Speakers Bureau; Kedrion: Consultancy; Octapharma: Consultancy; Pfizer: Consultancy, Research Funding, Speakers Bureau; Sanofi: Consultancy; Hema Biologics: Consultancy; Grifols: Consultancy; CSL Behring: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau. Stasyshyn: CSL Behring: Consultancy, Research Funding; Novo Nordisk: Consultancy, Research Funding, Speakers Bureau; Octapharma: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; Grifols: Consultancy, Speakers Bureau; Shire: Consultancy. Wang: Octapharma: Other; Pfizer/Spark: Other: clinical trial investigator; uniQure: Consultancy, Other: Clinical trial investigator; Hema Biologics: Consultancy, Other: Clinical trial investigator; Takeda: Consultancy, Other: Clinical trial investigator; Genentech: Consultancy, Other: Clinical trial investigator; Novo Nordisk: Consultancy, Other: Clinical trial investigator; CSL Behring: Consultancy, Other: Clinical trial investigator; Bioverativ: Consultancy, Other: Clinical trial investigator; Bayer: Consultancy, Other: Clinical trial investigator; BioMarin: Consultancy, Other: Clinical trial investigator. Windyga: Alnylam Pharmaceuticals: Research Funding; Novo Nordisk: Research Funding, Speakers Bureau; Octapharma: Research Funding, Speakers Bureau; Rigel: Research Funding; Roche: Research Funding, Speakers Bureau; Sanofi/Genzyme: Research Funding, Speakers Bureau; Shire/takeda: Research Funding, Speakers Bureau; Sobi: Research Funding, Speakers Bureau; Alexion: Speakers Bureau; CSL Behring: Speakers Bureau; Werfen: Speakers Bureau. Alexander: HEMA Biologics: Consultancy, Ended employment in the past 24 months. Al-Sabbagh: LFB: Current Employment. Bonzo: LFB: Current Employment. Macie: HEMA Biologics: Current Employment. Mitchell: HEMA Biologics: Consultancy, Current Employment. Wilkinson: GLOVAL, LLC: Consultancy. Shapiro: Genentech: Other: Advisory board fees, Research Funding, Speakers Bureau; Glover Blood Therapeutics: Research Funding; Kedrion Biopharma: Research Funding; Daiichi Sankyo: Research Funding; Bioverativ (a Sanofi company): Other: Advisory board fees, Research Funding; Takeda: Research Funding; Novo Nordisk: Other: Advisory board fees, Research Funding, Speakers Bureau; Octapharma: Research Funding; OPKO: Research Funding; Sangamo: Other: Advisory board fees, Research Funding; Prometric BioTherapeutics: Research Funding; Pfizer: Research Funding; Sigilon Therapeutics: Other: Advisory board fees, Research Funding; Novartis: Research Funding; BioMarin: Research Funding; Agios: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal